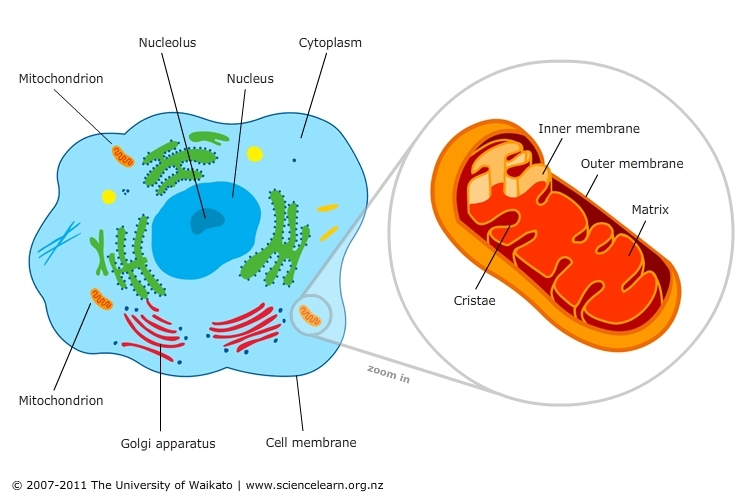
Mitochondrial dysfunction is the cause of muscular inflammation, which is the most common complaint treated in the Chiropractic setting. The symptoms of this dysfunction can range much further than the muscular system, as we will discuss. The mitochondrion is an organelle within most cells of the body. They can range from 100,000 to 600,000 per cell. The mitochondria are referred to as the powerhouse of the cell. This is because it is the place where sugar, fats, and proteins are converted into the usable form of energy, called ATP. The more metabolically active a cell is, the more mitochondria it will contain, such as liver and muscle cells. Only a few cells, such as red blood cells and some cells of the eye, have no mitochondria.
Mitochondrial dysfunction resulting in Oxidative Stress is an area of heavy research. The process of aging is driven by oxidative stress, from the wrinkles on your face and grey hair to Parkinson's and Dementia. The spectrum of illnesses related to oxidative stress is vast, and knowledge of its mechanisms is growing rapidly.
We are going to break down the process of ATP production and then discuss the importance of mitochondria. We will start with Glycolysis, then the Krebs Cycle, and finish with the most important process, the Electron Transport Chain.
Glycolysis
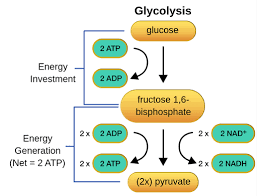
Small amounts of ATP can be produced by the breakdown of glucose in the cell outside the mitochondria in a process called glycolysis.
During glycolysis, enzymes break down glucose to pyruvate, which requires 2 ATP. Once the glucose is broken down, 4 ATP are produced, leaving 2, and therefore not being very efficient. The pyruvate and the NADH move the mitochondria for the large energy production in the Krebs Cycle and the Electron Transport Chain. Important to note that this first process, glycolysis, does not require anything other than sugar and a couple of ATP, and the only byproducts are more than 2 ATP, 2 Pyruvate, and 2 NADH. The next steps in the mitochondria are dependent on the presence of oxygen. Therefore, we refer to glycolysis as anaerobic respiration (no oxygen required) versus the Electron Transport Chain being aerobic respiration (requires oxygen).
Krebs Cycle
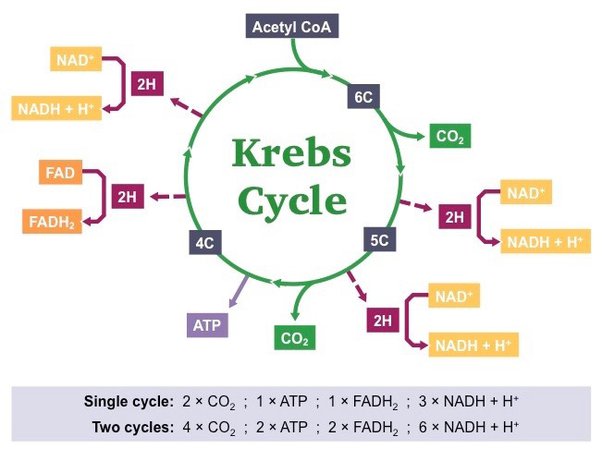
The Krebs Cycle, also called the citric acid cycle, gets very confusing, but we will make it simple. First, we must note that it doesn't start with pyruvate, but an important molecule called Acetyl-CoA, which helps in energy production. Second notice, no oxygen is necessary for this cycle of sugar metabolism, even though it occurs in the mitochondria. For every glucose broken down, 2 pyruvate molecules enter this cycle, so it will happen 2 times per glucose. During this process, carbon dioxide CO2 is produced, which is an unwanted byproduct that is carried by the blood to the lungs, eventually to be exhaled. The benefit of this cycle is the production of another 2 ATP, but also 8 NADH and 2 FADH. So far, only a net of 4 ATP has been produced (not very much), but the 10 NADH and 2 FADH will be very important in the next step, the Electron Transport Chain.
Another important note is that some cells in the body can use fat and protein by breaking them down to Acetyl CoA to enter the Krebs Cycle, skipping glycolysis (carbohydrate metabolism) to produce ATP. Between meals, the heart muscle uses fat for 90% of its energy needs and drops to 60% right after a meal. During rest and moderate activity, skeletal muscle uses fat as its primary energy source for ATP. Only during heavy exercise does glucose predominate over fatty acids. In contrast, some cells, such as red blood cells (which have no mitochondria), and cells of the retina/lens( also no mitochondria) of the eye, prefer glucose (only using glycolysis). The central nervous system prefers glucose but can operate on ketones from fat as an energy source. Ketones are produced in the liver by breaking down fatty acids, but have a lag time during extended fasting.
Electron Transport Chain
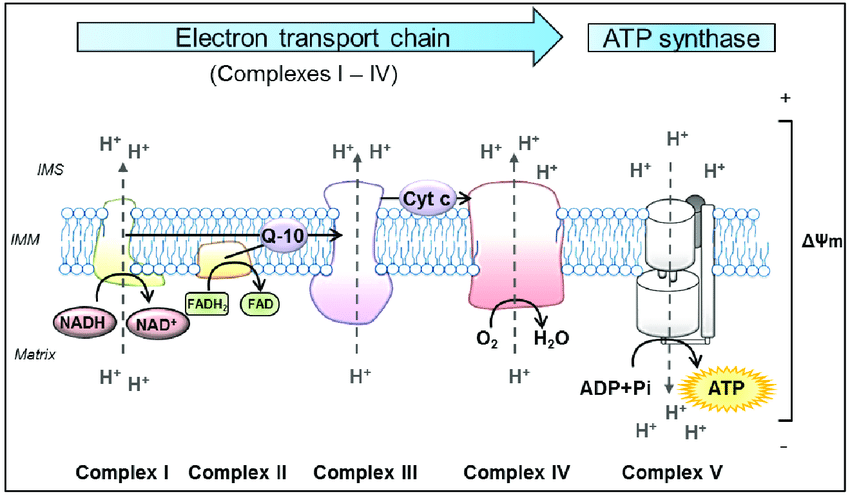
The electron transport chain is how the majority of ATP is produced and is dependent on oxygen and light (will discuss at the end). Electron transport is aerobic respiration. This will be a little complicated, but we will summarize at the end, and try to follow it. The energy stored in the NADH and FADH from glycolysis and Krebs is delivered to the system as electron donors to the inner mitochondrial membrane IMM Complex I and II (follow illustration above). The protein complexes are embedded in a membrane that separates the inside matrix of the mitochondria from a gap space called the inner membrane space IMS. The electrons are moved through the different protein complexes to induce the movement of positively charged protons into the IMS between the outside membrane of the mitochondria and the IMM. This separation of charge created by moving protons to the IMS is the potential energy used to create ATP at Complex V. During this process in Complex IV, Oxygen is required as the end donor of electrons, creating water. If everything is efficient, which it never is all electrons are donated to oxygen. The movement of the electrons through the protein complexes causes phase transitions of the protein/water dynamics, moving the protons to the inner membrane space.
NADH will create 3 ATPs by donating its 2 electrons to Complex I, and FADH will create 2 ATPs by donating its single electron. During this process of consuming 1 Glucose molecule, 10 NADH produced will yield 30 ATP, and 2 FADH molecules will yield 4 ATP for a total of 34 ATP produced in the electron transport chain during aerobic respiration. Anaerobically, 2 ATP were produced in Glycolysis and another 2 in the Krebs Cycle for a total yield of 38 ATP per glucose. The majority of energy produced in the Electron Transport Chain was dependent on the movement of charge via electrons through proteins to move protons (positively charged), creating potential energy needed for Complex V to produce ATP.
Very important to note that electrons are transferred to 2 Intermediate complexes, Q-10 and Cytochrome C (see the figure above, Q-10 and Cyt C) between the large protein complexes. These 2 complexes are mobile within the inner mitochondrial membrane. The first, Q-10, also called coenzyme Q 10 is a very strong antioxidant. Electrons from Complex I and II donate electrons to Q-10, which it then carries to Complex III. Coenzyme Q-10 deficiency is a very common issue that will be discussed later. The second, Cytochrome C, accepts electrons from Complex III and delivers them to Complex IV. Complex IV, as you can see above, has another vital step in the process. Not only does it pump protons into the IMS, but it is also responsible for terminating the electron flow by adding electrons to oxygen to form water. This controlled termination prevents those electrons from escaping and becoming reactive oxygen species. Cytochrome Oxidase is the enzyme within complex IV responsible for this final step of electron transport. Cytochrome C and Cytochrome Oxidase are the most studied areas of the electron transport chain concerning disease processes. These proteins are in the heme family, carrying the same type of iron bond protein arrangement as hemoglobin in our red blood cells. Cytochrome C has more functions in the electron transport chain than just electron transfer. It controls the rate at which electrons flow to the final 2 complexes and is deemed the rate-limiting step of ATP production. Cytochrome C not only controls the rate of ATP production but also monitors the efficiency of the first 3 complexes. If the first 3 complexes are losing electrons back to the matrix, a sign of a major malfunction in the mitochondria. Cytochrome C can induce cell death to protect nearby cells from toxicity.
Mitochondrial Dysfunction: Electron Transport Disorders and Disease
Complex I, III, and IV are the sites where electrons are transported (Via Quantum Tunneling) to pump protons into the IMS. This is very important because at these 3 complexes, bottlenecking of electron flow can occur. At these locations, the electrons can escape back into the matrix, creating dangerous compounds called reactive oxygen species (oxidants). Oxidants can damage proteins, DNA, and cellular lipids. This issue is dealt with in the mitochondria, which produce high amounts of melatonin (a very strong antioxidant, not just for sleeping) that covers 95% of the antioxidant needs of the mitochondria under normal circumstances. This is why it is advisable to add antioxidants to the diet to control aging, cancer, and inflammatory/degenerative diseases. Under normal circumstances, .2-2% of electrons transiting these complexes escape and create reactive oxygen species. If more than usual electrons escape, it creates oxidative stress on the cell, and if it is widespread can cause oxidative stress-related illness. If the levels get high enough, Cytochrome C starts the process of cell destruction.
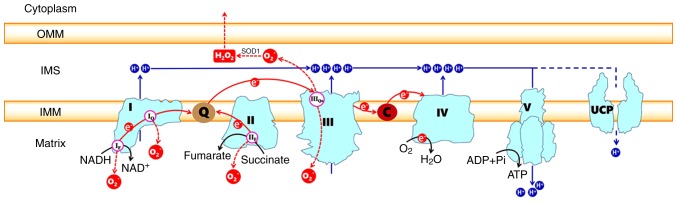
Causes of electron transport disorders rarely originate in Complexes I-V (rare genetic disorders deadly soon after birth) but are more likely due to Q-10 and Cytochrome dysfunction. Complexes I, II, and III are the site of electron escape leading to reactive oxygen species production (See red circle O2 escaping into the matrix). In this figure, you can see electrons flow from Complex I and II to Q (Coenzyme Q-10), then from Q, they flow to Complex III. After flowing through III, they go to C (Cytochrome C). Cytochrome C then sends the electrons to complex IV. Cytochrome oxidase in complex IV finally donates the electrons to Oxygen (O2) to make water. The cause of the escape is believed to be the result of Q-10, Cytochrome Oxidase, or Cytochrome C not accepting the flow of electrons. The Complexes I-IV perform Quantum Tunneling of the electrons to push protons (blue H circles) via water/protein phase transitions into the IMS, building potential energy needed for Complex V to make ATP.
Q-10 (Coenzyme Q-10) deficiency, not dysfunction, results in electron escape and oxidative stress. Q-10 is not produced in the mitochondria. Co Q-10 is produced in the liver and is minimally supplemented by dietary intake. Cholesterol-lowering medications such as statins inhibit liver production of Q-10, resulting in moderate/severe Oxidative Stress Disorders. Supplementation is vital for people on these medications. Other health conditions of the liver can also cause a deficiency of Q-10.
Cytochrome C and Cytochrome Oxidase are produced by the mitochondrial DNA and have very rare genetic mutations, which limit the life span soon after birth. Decreased Cytochrome function is a very common cause of slowing of the electron transport chain. If Cytochrome C is not rapidly moving electrons between Complex III and IV, it increases the likelihood of electron escape and reactive oxygen species formation earlier in the chain. If Cytochrome Oxidase does not accept the electron and convert it to water, electrons can escape and also create reactive oxygen species.
Cytochromes are two of many light-absorbing molecules (chromophores) in the body. Light-absorbing molecules will absorb the energy of a particular wavelength of light, transforming their structure to the excited state. In the excited state, a chromophore is very efficient at transferring electrons.
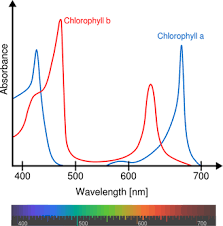
Chlorophyll is a chromophore in plants responsible for photosynthesis. Photosynthesis is the process of ATP production in plants using light as the energy to move electrons in their electron transport chain. Plants absorb blue and red light frequencies to excite their chromophores to an excited state for electron movement. They reflect green, as you can see in the above chart, so we see them as green.
Cytochrome C and Cytochrome oxidase absorb light in the red spectrum, exciting the molecules in the Heme structure, optimizing their ability to transfer electrons. Any alterations in this efficiency to transfer electrons greatly increase reactive oxygen species within the mitochondria. Research has proven that Cytochrome dysfunction can lead to many diseases such as type II diabetes, obesity, Parkinson's Disease, Alzheimer's, cardiovascular disease, and autoimmune diseases.
Many studies are also showing that asthma and allergies are linked to increased reactive species production. The cause originates either in mitochondrial dysfunction or xenobiotics, creating oxidative stress through other immune system mechanisms. Xenobiotic substances are chemical substances that are foreign to animal life, such as plant constituents (pollens or oils), drugs, pesticides, cosmetics, flavorings, fragrances, food additives, industrial chemicals, and environmental pollutants. These substances have reactions poorly understood at this point. Research is finding that these substances create oxidative stress on the cells soon after exposure. It is not known if it is the result of mitochondrial alterations or is detoxification/immune-related.
Research is also linking Cytochrome functional alterations induced by radiofrequency exposure. The studies being performed are on different frequencies, ranging from low-frequency electromagnetic fields in household electricity up to very high frequencies in the telecommunication and wireless internet industries. The findings all conclude that oxidative stress does occur from the build-up of reactive oxygen species within the mitochondria, resulting in a variety of cellular alterations that can lead to illness. The mechanism of radiofrequency alterations on Cytochrome C and Cytochrome oxidase is poorly understood, but it is theorized that the vibrational state within the vital Heme molecule is altered, decreasing its ability to function as an electron acceptor/donor in the electron transport chain. This theory is supported by other research on the effects radio frequencies have on red blood cells. Similar research shows radiofrequency exposure changes the structure of the hemoglobin molecule (which has the same Heme structure as Cytochromes), resulting in decreased ability to carry oxygen in the red blood cells.
Theory of Mitochondrial Origins is the Key
As we have discussed above, light (photon energy at specific wavelengths) is necessary for mitochondrial function. To understand why an organelle in almost every cell of our body requires light, we must first understand the origin of the mitochondria. The origin of mitochondria in complex cellular organisms starts with a theory of symbiosis (mutually beneficial relationship of two organisms). The early single-celled organisms are divided into two categories: Prokaryotes and Eukaryotes. Prokaryotes are cells with very little complex structure, lacking a central nucleus, cytoskeleton, or other organelles. Early eukaryotes possessed a central nucleosome, organelles, and a complex cytoskeleton. It is believed that at some point, a eukaryotic cell engulfed a prokaryotic cell to hijack it for more energy. The process was slow initially, binding to it and then slowly merging it into itself. The engulfed prokaryote is widely held to be the origin of the mitochondria. Mitochondria retain all the characteristics of a prokaryotic cell, encoding their DNA and proteins and undergoing self-replication. Early cells contained pigments that allowed energy conversion from light photons to be used to generate a usable form of energy, ATP, similar to photosynthetic plants. Mitochondria produce an iron-containing pigment called Heme. These pigments are still present as the Cytochromes within the mitochondria, continually absorbing photon energy in the red and near-infrared spectrum. This energy excites the electrons of the cytochromes to facilitate the Redox (oxidation/reduction) reactions performed during cellular energy production. The Heme structure within the cytochromes is responsible for the oxidation/reduction capacity of the cytochromes.
The mitochondrial DNA encodes the production of the Heme molecules. Heme structures produced in the mitochondria are used in other reactions outside of energy production. Heme binds to other protein complexes such as hemoglobin in red blood cells, multiple proteins that function in biochemical synthesis and degradation processes, and proteins that detoxify hydrogen peroxide in cells. Cytochrome P-450, a Heme protein in the liver, detoxifies the body from xenobiotic exposure. This same enzyme is found in the brain, serving as a detoxifier and regulator of neurotransmitter expression. It is highly concentrated within the anterior pituitary, which controls all hormone activity in the body. For this reason, it is also believed to affect hormone production and balance.
This symbiotic relationship, which occurred a billion years ago, gave the advantage of increased energy production. Increased energy production allowed for cellular specialization, leading to the formation of complex multicellular organisms. Through the production of Heme, the mitochondria not only gave eukaryotic cells an energy advantage but also allowed oxygen transport via red blood cells to develop and multiple other biochemical pathways to develop. In return, the early prokaryotic cell got the benefit of protection and nourishment. Each eukaryotic cell has between 100,00 and 600,000 mitochondria living inside its protective barrier, receiving all the nutrients it needs.